Newborn Screening
Owing to the rarity of Metachromatic Leukodystrophy (MLD) and other very serious conditions, many children are misdiagnosed or diagnosed at a point where the disease has already progressed too far for cutting-edge treatments such as gene therapy to be of benefit.
ArchAngel is dedicated to ensuring that Metachromatic Leukodystrophy (MLD) is added to the UK’s Newborn Bloodspot Screening (NBS) Programme to guarantee timely diagnosis. The Trust has established an MLD NBS Steering Group of highly specialist scientists and clinicians to carry out the work required and provides the group secretariat. Progress on the application to date is as follows:
In December 2021 the steering group submitted a formal application to the National Screening Committee (NSC).
In April 2022 the UK NSC Secretariat confirmed it would commission an ‘evidence map’ to further examine the case for MLD.
In June 2023 the NSC published an evidence review, stating that “the available evidence on screening test accuracy and cost-effectiveness, though limited, is promising and warrants further review. It found that the volume and type of evidence related to the benefits and harms of treatments in presymptomatic patients with MLD is sufficient to justify a more in-depth review of the evidence”.
June 2023 MLD was placed in the standard the 3-year review cycle. The case will therefore be reviewed in 2026, unless significant new evidence is available prior to that date, in which case the steering group could apply for a rapid review. The steering group is actively working on producing and gathering further evidence to meet the NSC’s requirements.
In February 2024 Wu et al. published very favourable results of a pre-pilot study of the performance of a test developed by Prof. Michael Gelb in the US. ArchAngel is extremely grateful to the high number of UK MLD families who made this vital research possible by authorising use of their MLD affected child’s bloodspot card to provide accurate control material. The next stage of this work is a pilot study, which is currently in planning and seeking necessary permissions.
- In May 2025 the UK NSC opened a 3-month public consultation into proposed NBS for MLD, with guidance that the NSC would NOT support recommendation to approve. The NSC received 501 responses expressing overwhelming support for the approval of screening. We sincerely thank everyone who took the time to express their professional and personal opinions in response to the consultation.
- In January 2026 the NSC published their evidence summary findings, with a confirmed recommendation NOT to approve screening. The NSC indicated the next evidence review will take place in 2029/2030. This is entirely unacceptable to our community. Since Libmeldy was approved in 2022, only 6/37 children diagnosed have been eligible for treatment. The remaining 31 children will face unimaginable suffering and certain premature death. With the next NSC review in 3-4 years hence, another 30-40 children will likely be left to die. We are challenging this decision at the highest possible levels.
Our MLD NBS expert steering group has identified a number of methodological flaws in the review process and will be meeting with the NSC in the coming weeks to challenge both the process and the resulting negative recommendation. We also hope to learn more about the EquipoISE – a multi-condition ‘in service evaluation’ project – which the NSC has recently announced, citing MLD for potential inclusion.
Alongside this, ArchAngel, MPS Society and MLD Support UK continue to work together to publicly campaign against the NSC decision and to highlight the multiple failings of the UK system. We have a number of engagements with MPs and the Ministry of Health, as well as some powerful press support. Please see our social media channels for further details.
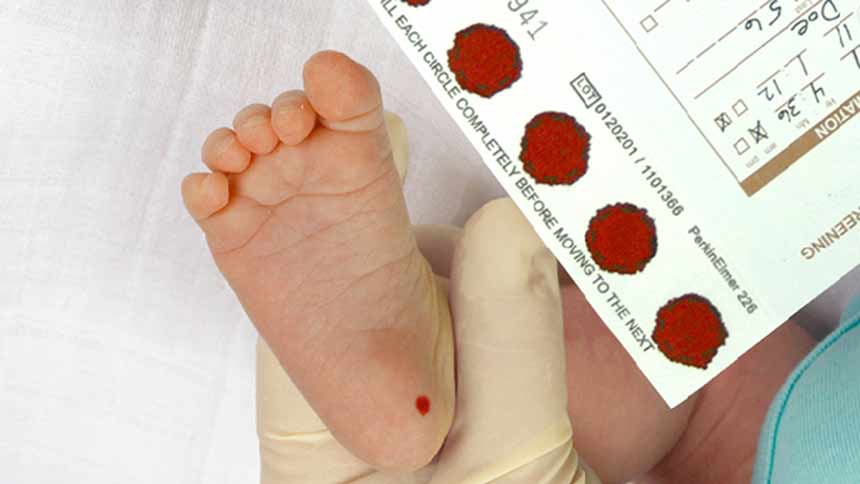
Rare Diseases disproportionally affect children and diagnostic delays result in unnecessary suffering and undue burden on the health service. Newborn screening has the power to dramatically improve children’s lives, by facilitating early access to treatment, maximising the chance for eligibility for treatments and reducing the chance for serious effects of disease prior to treatment.
In the United Kingdom (UK) babies are screened via the heel-prick test (also known as the blood spot or Guthrie test) at 5 or 6 days old. A midwife visits the family home and takes a blood sample by making a small prick on the baby’s heel. The blood sample is then sent to a laboratory, where it is analysed or ‘screened’ for 9 rare disorders. Many other high-income countries screening for a much greater number of conditions. However, the current system for accepting new disorders onto the UK programme remains a very complex and lengthy process. The criteria and standards for screening are set and monitored by the National Screening Committee and Public Health England.
England Rare Disease Action Plan
In February 2022, the DHSC published the England Rare Disease Action Plan containing an important commitment to improving newborn screening, in line with the key policy changes which the Collaborative has been calling for:
You can read the full England Rare Disease Action Plan here:
The Collaborative will continue to work with the DHSC on the detail of the above promises at every opportunity and hope that there will be more positive news to share in the coming months.
In the meantime, exciting developments in Genomic screening has raised many questions about how and when this will fit into the current programme, the attached briefing paper outlines the considerations which we are exploring with experts:
Click here to download NBS Genomics Briefing Paper
In 2020 ArchAngel MLD Trust brought together 13 principal rare disease patient organisations, plus Genetic Alliance UK, to form a new UK NBS Collaborative, representing over 500 rare diseases and thousands of patients. The Collaborative [link] actively campaigns for key policy changes which would allow for the appropriate and efficient expansion of the UK NBS programme, in order to improve the prospects of all future MLD affected patients and many other rare diseases where treatments and tests are available.
The Collaborative was inspired by the important work of Pat Roberts, who has been dedicated to this field for over 20 years through close family experience of another IMD, Krabbe disease. She founded both the Save Babies Through Screening Foundation UK and the Patient Advocates for Newborn Screening Group, and has worked extensively alongside scientists, Public Health England and numerous other key health professionals on the extension of the newborn screening programme in the UK for all inherited metabolic disorders. ArchAngel MLD Trust is incredibly lucky to have Pat as Director of Newborn Screening Project.